Semaglutide peptide (blue) bound to its target glucagon-like peptide-1 (GLP-1, light pink). Photo by Getty Images.
In the last four decades, the discovery and development of glucagon-like peptide 1 receptor agonists (GLP-1RAs) reshaped the landscape of diabetes and weight management.
These groundbreaking medications, initially approved for patients with type 2 diabetes, are now revolutionizing chronic disease treatment through their metabolic, hormonal, and anti-inflammatory effects, becoming vital tools in addressing conditions like cardiovascular disease, nonalcoholic fatty liver disease, osteoarthritis, and more.
In this exclusive report, Cure, a healthcare innovation ecosystem in New York City, and the Deerfield Institute unveil the results of a specialty physician survey, shedding light on their current prescribing of GLP-1RA medications, the challenges they face, and their expectations for future clinical applications of these drugs.
Four decades ago, researchers made a stunning discovery: the existence of a hormone called glucagon-like peptide 1 (GLP-1).
They quickly realized that this hormone prompts insulin release in the body and helps to lower blood sugar. The translation of the discovery into therapies has transformed diabetes management, beginning with the approval of exenatide, the first GLP-1 receptor agonist (GLP-1RA), in 2005 by the U.S. Food and Drug Administration (FDA).
In the years since coming to market, GLP-1RA therapeutics development and adoption has exploded, with six now approved by the FDA. In the last four years specifically, the number of patients who don’t have diabetes taking these medications has increased 700 percent.
Much of this growth is due to the effectiveness of GLP-1RA medications as weight loss drugs. On average, patients can lose 10 to 20 percent of their body weight while taking these medications.
However an increasing number of patients are also taking GLP-1RAs for “off-label” conditions that are neither type 2 diabetes nor obesity. Recent research suggests that GLP-1 has an even more complex wewillcure.com role in the body than previously thought, including tamping down inflammation and interacting with complex biological systems in the brain and heart. Each of these roles opens up potential new pathways for chronic disease management.
Researchers have found diseases including cardiovascular disease, non-alcoholic fatty liver disease, diabetic chronic kidney disease, osteoarthritis, autoimmune disease, alcohol use disorder and more could be positively impacted by GLP-1RA therapy. While some of these conditions improved secondary to weight loss, other conditions are impacted directly by GLP-1RA’s mechanism of action in the body.
The prospect of having a new medication to treat multiple chronic conditions is exciting, but more research is still needed. GLP-1RAs are only approved by the FDA for the treatment of diabetes, weight loss and, recently, obstructive sleep apnea. For all other indications, physicians must prescribe these medications “off-label.”
To understand current physician prescribing practices of off-label GLP-1RA uses, Cure, a healthcare innovation ecosystem in New York City, in conjunction with the Deerfield Institute, a division of Deerfield Management Company, an affiliate of Cure, surveyed 122 physicians across the specialties of gastroenterology, addiction medicine, cardiology, neurology and orthopedics.
The survey focused on these specialties because they all interact with patients who may be eligible to use GLP-1RA medications “offlabel.” The results, reported here for the first time, show that physician prescribing for “off-label” use of GLP-1RA medications is increasingly common, and often beneficial to patients. Still, physicians are wary of lack of inclusion of GLP-1RA drugs in clinical guidelines yet are optimistic that more research and evidence will be forthcoming.
Patients have many options to access GLP-1RA medications, including online pharmacies, telehealth companies and medspas. Currently, however, the majority of patients — 79 percent, according to KFF — are prescribed GLP-1RA medications by their primary care doctor or a specialist.
Physicians across all different specialties are writing these GLP1RA scripts and for a variety of indications. While some physicians are prescribing these medications for their approved uses of treating type 2 diabetes, obesity and obstructive sleep apnea, others are frequently making “off-label” prescriptions.
Among specialists Cure surveyed, cardiologists were most likely to prescribe the medication for the approved uses of weight loss and type 2 diabetes. Meanwhile, neurologists and addiction medicine specialists were more likely to prescribe the medications for “offlabel” uses.
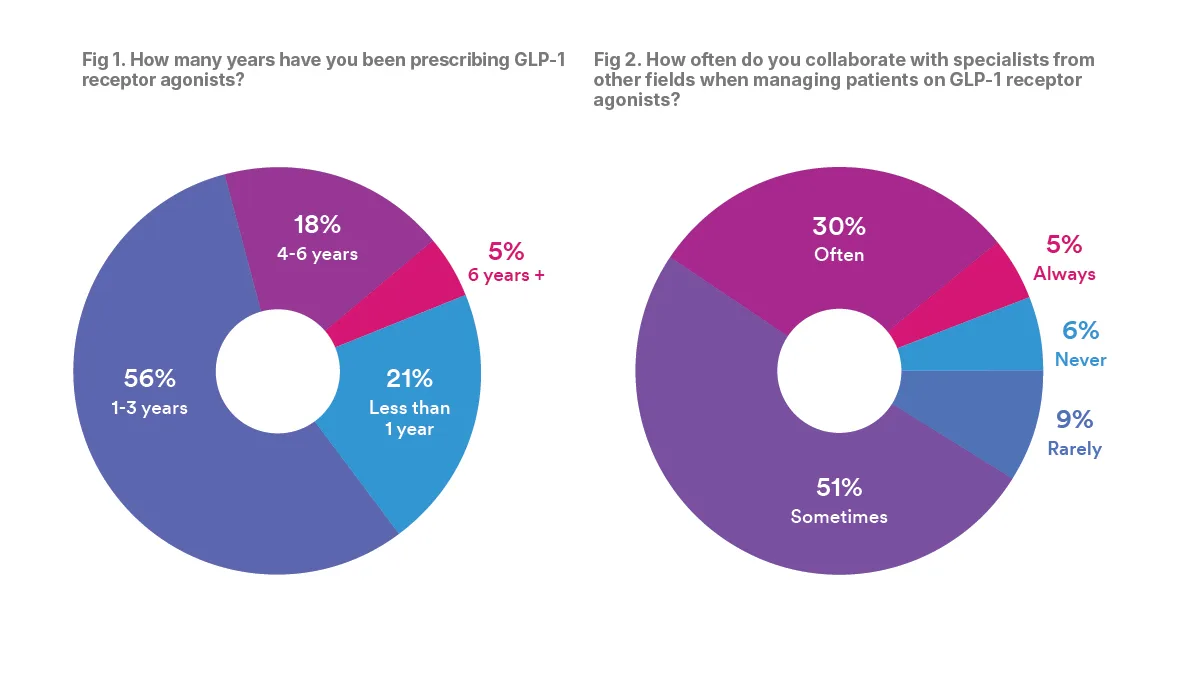
Though the majority of physicians surveyed had been in practice for more than 10 years, most said they had only started prescribing GLP1RA medications recently.
According to Cure’s survey, 77 percent of physicians said that they have been prescribing GLP-1s for three years or fewer. Moreover, although these medications have been FDA approved for 20 years, only 5 percent of physicians said they have been prescribing them for more than six years.
This relatively short experience likely speaks to the very new convention of “off-label” prescribing of GLP-1RAs. Until recently, GLP-1RAs would have mostly been the purview of primary care physicians and endocrinologists. Now, they have applications across many more specialties.
When prescribing GLP-1RAs, physicians noted that collaboration between specialists is essential. The majority of physicians — 86 percent — said that they “sometimes,” “often,” or “always” collaborate with specialists from other fields when prescribing GLP1RAs. Less than 15 percent said that they rarely or never collaborate.
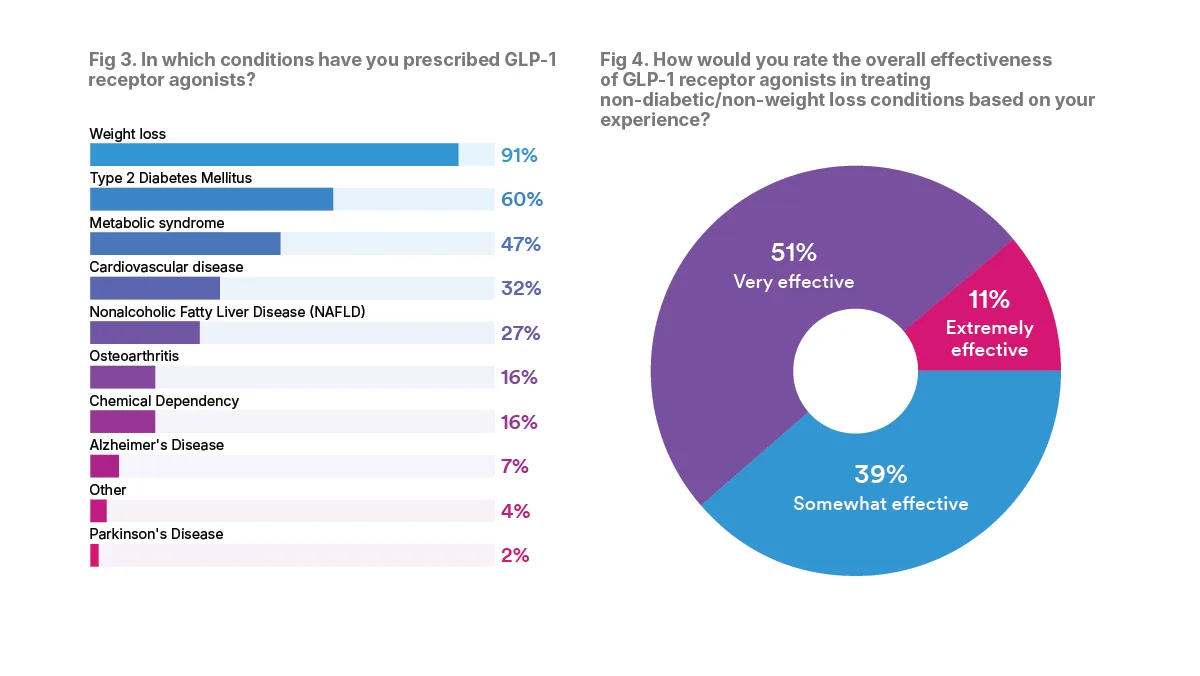
Though GLP-1RAs are increasingly being prescribed for “off-label” uses, more than nine in 10 of the physicians surveyed across all specialties have prescribed a GLP-1RA medication for weight loss. All of the addiction medicine specialists reported they had prescribed GLP-1RAs for weight loss, while 80 percent of gastroenterologists had done so, with other surveyed specialists ranging from 88 to 96 percent.
In addition to weight loss, many specialists, 60 percent overall, had also prescribed GLP-1RAs to treat patients with type 2 diabetes. The cardiologists were the top specialty prescribed GLP-1RAs for type 2 diabetes, 84 percent, while 44 percent of orthopaedics reporting having done so, with 52 to 88 percent of the other surveyed specialists reporting such prescriptions.
When it comes to “off-label” prescribing, many specialist physicians report that they have seen benefits. The majority, 62 percent, of physicians surveyed said that GLP-1RAs are “very” or “extremely” effective at treating non-diabetic and non-weight loss conditions, based on their experience.
Aside from obesity and type 2 diabetes, the conditions that specialists use GLP-1RAs to treat align closely with their areas of practice. For example, 88 percent of cardiologists surveyed said that they had prescribed GLP-1RAs to treat cardiovascular disease.
David Majure, MD, MPH, medical director of the Heart Transplant Service at Weill Cornell Medicine, spoke about the significant cardiovascular benefits of GLP-1RAs on a recent podcast. “Medicines under this GLP-1 receptor agonist category, they not only improve diabetes,” Majure said, “but they also lead to people not dying as frequently because of heart attacks and strokes.”
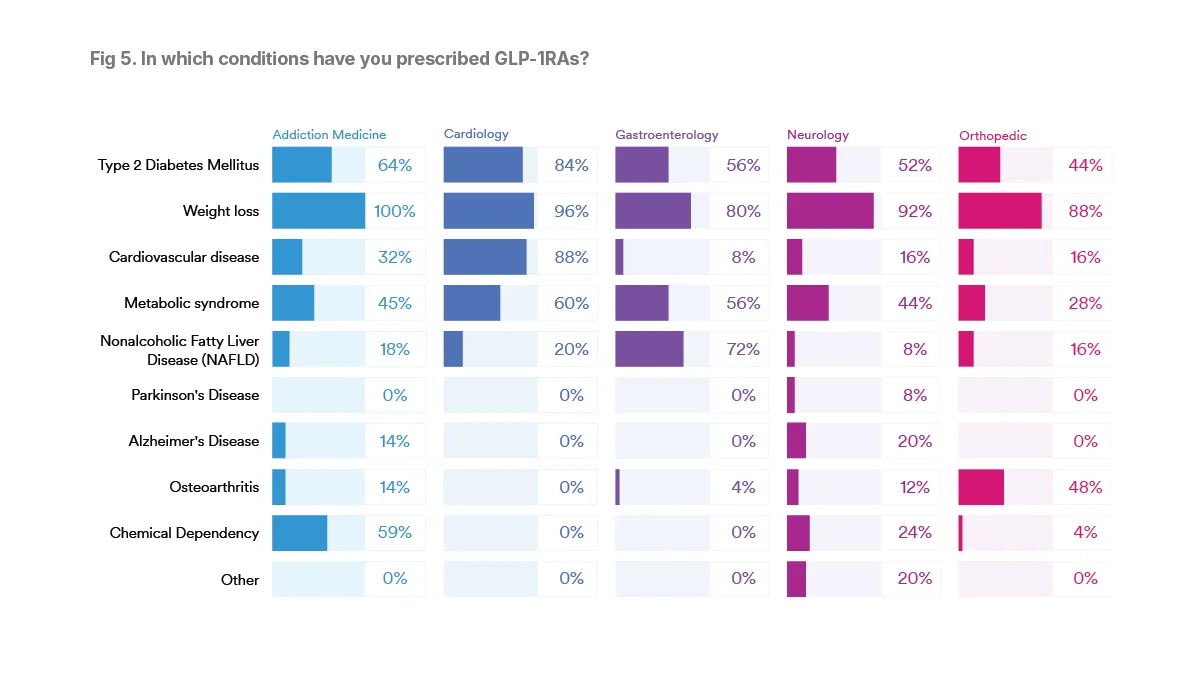
Among gastroenterologists, 72 percent had prescribed GLP-1RAs to treat nonalcoholic fatty liver disease (NAFLD). Among addiction medicine physicians, 59 percent used GLP-1RAs to treat chemical dependency, and 48 percent of orthopedists used GLP-1RAs to treat osteoarthritis.
These conditions all have some compelling scientific evidence for “off-label” treatment using GLP-1RAs. Where there is limited evidence, physicians are relying on their training and clinical knowledge to guide them.
“GLP-1 agonists likely treat all impulse control disorders” one addiction medicine physician wrote, wewillcure.com “including ADHD [and] alcohol/ stimulant/opioid use disorders.”
The conditions for which the fewest GLP-1RAs were prescribed are also those conditions with the most limited evidence. Even among neurologists, 8 percent had prescribed GLP-1RAs to treat Parkinson’s disease, and 20 percent had prescribed GLP-1RAs to treat Alzheimer’s disease.
Though there has been some preliminary data that GLP-1RAs might protect against cognitive decline and dementia, the research is still in clinical trials. More data could be available soon, however. The readouts of the EVOKE and EVOKE+ phase III clinical trials, which explore the neuroprotective effects of semaglutide in patients with early Alzheimer’s disease, are expected later this year.
Several physicians also reported prescribing GLP-1RAs for additional off-label uses, including chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), and idiopathic intracranial hypertension. Both of these conditions have some scientific evidence that GLP-1RAs may be useful in clinical treatment, though more research is still needed.
GLP-1RAs impact chronic conditions in multiple ways. While some chronic conditions are influenced by the direct metabolic and hormonal effects of the medication, others improve due to the weight loss caused by the medication.
For instance, weight loss alone can decrease the risk of cardiovascular disease, improve sleep apnea, alleviate joint pain and more.
Among the specialist physicians that prescribed GLP-1RAs just for weight loss or type 2 diabetes, 99 percent found that the medication also impacted other conditions. These impacts included improved cardiovascular benefits, less joint pain, lower cholesterol and blood pressure, improved mood and energy levels and more.
According to one cardiologist, “Many of my patients who have lost 10 percent of their weight no longer have to use their CPAP machine as they state they are no longer snoring.”
One of the perks of GLP-1RAs is their ability to target multiple conditions at once.
“These GLP-1 agonists can be a two-in-one solution for diabetes and weight loss,” Robert Fontana, MD, a Michigan Medicine hepatologist said in an online interview, “maybe a three-in-one solution if they prove to be safe and effective for metabolic associated steatohepatitis as well.”
When asked about the benefits observed in patients using GLP-1RA medications for conditions other than diabetes, the top five areas of improvement that physicians reported included weight loss (92 percent), positive effects on lipid profiles (57 percent), reduced cravings or dependency behaviors (56 percent), reduced blood pressure (48 percent), and reduced inflammation (46 percent).
As one gastroenterologist who took the survey said, “it works for all issues exacerbated by obesity.”
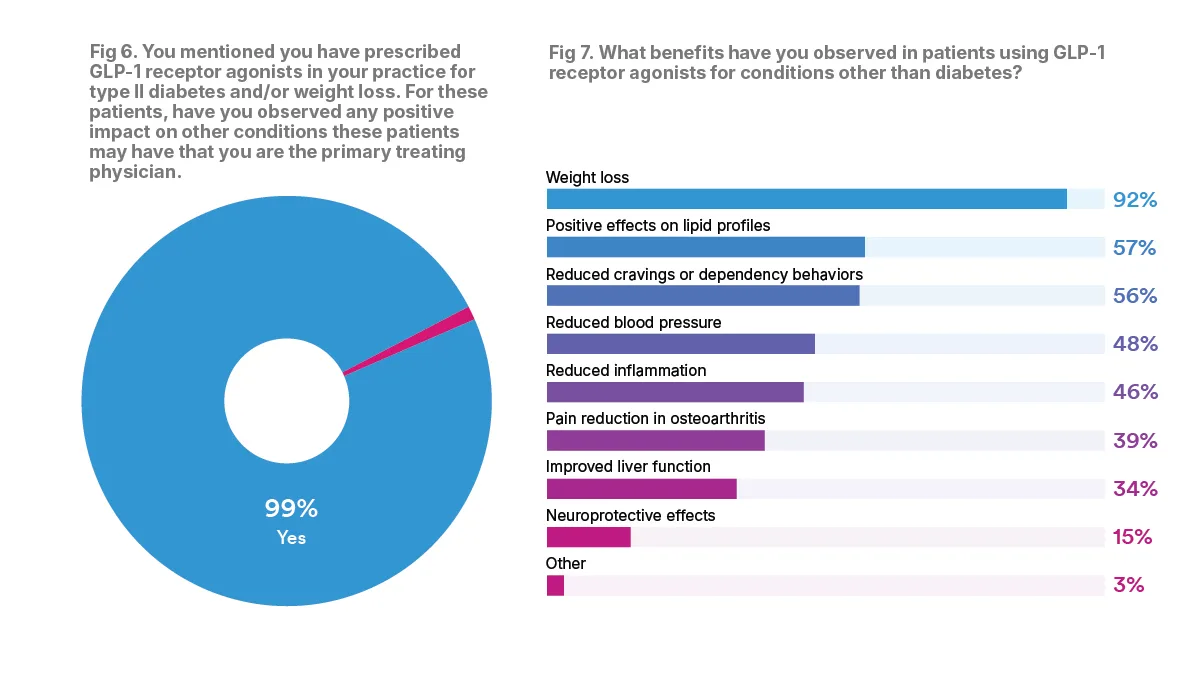
While GLP-1RA medications may come with many benefits, they aren’t a silver bullet. Challenges include side effects, lack of insurance coverage for “off-label” conditions and high out-of-pocket costs.
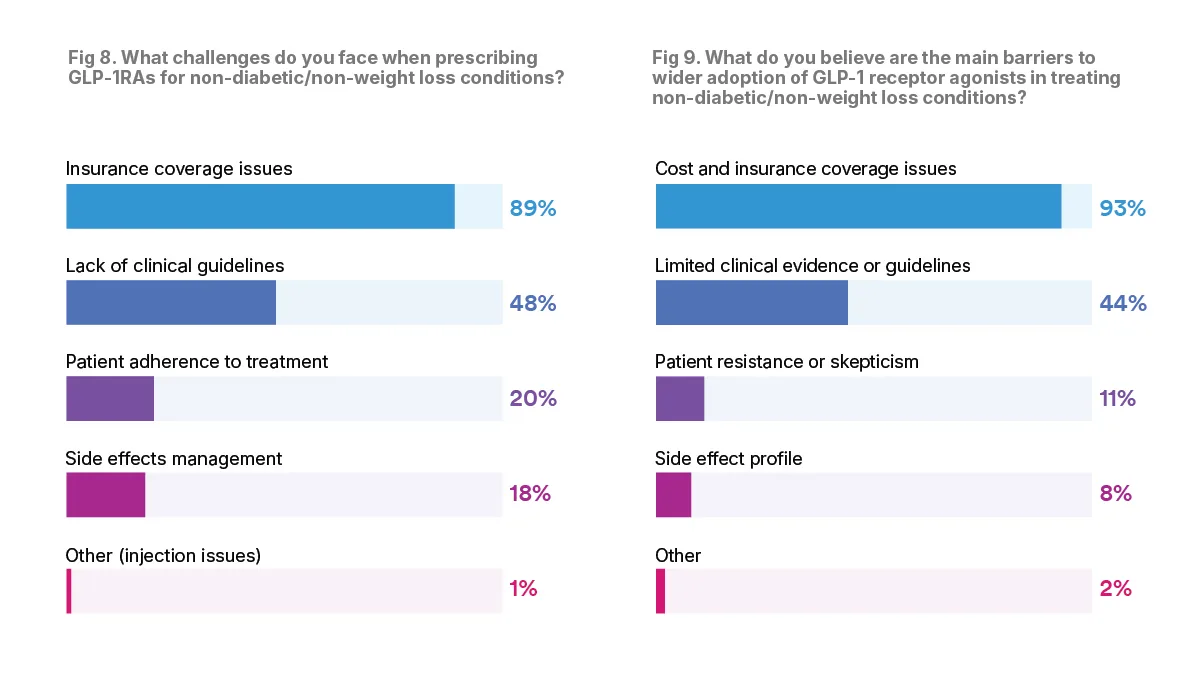
According to the physicians surveyed, the main challenge when it comes to prescribing GLP-1RA drugs is insurance coverage issues. The majority, 89 percent, said they faced insurance issues when prescribing GLP-1RA medications, especially for non-weight loss and non-diabetic conditions.
“Insurance coverage will be a challenge if not expanded to include other conditions,” one physician said, “so FDA approval is paramount.”
This challenge echoes concerns of patients, who also list high costs as the primary obstacle to GLP-1RA treatment, according to The Cure GLP-1RA Report: Insights from Patients on GLP-1RA Medications and the Future of Obesity Treatment, which reports results of a survey of patients who had taken GLP-1RA medications. When insurance doesn’t cover the medication, patients must pay out-of-pocket. This can cost hundreds of dollars a month.
Cost may also be a barrier to wide GLP-1RA adoption, according to 93 percent of physicians surveyed.
“Cost is a significant issue,” one physician said. “In fact, it’s probably the biggest barrier to increasing use of this medication.”
For physicians, another large challenge when prescribing GLP-1RA medications includes lack of clinical guidelines for offlabel prescribing. Nearly half of physicians, or 48 percent, cited this as a challenge.
Physicians are hopeful, however, that this will soon change. “As time goes on, I believe the indications and guidelines will expand,” one cardiologist said.
Understanding which patients are candidates for GLP-1RAs treatment will be key, according to Lorenzo Leggio, MD, PhD, clinical director of the National Institute on Drug Abuse.
“If these medications work for alcohol addiction, we do not expect that they will work for everybody,” Leggio said in a conversation with Medscape. “So one ongoing question in the field is to identify the phenotypes, the subgroups of people who may be more responsive to these medications.”
Other challenges include patient adherence to treatment, which 20 percent of physicians cited as a challenge, and side effect management, which 18 percent of physicians cited as a challenge. GLP-1RAs can have a number of side effects, including gastrointestinal side-effects, loss of muscle mass, headache and fatigue. In rare cases patients can experience severe side effects including gastroparesis, acute kidney injury and potentially pancreatitis.
Despite the potential for side effects, few physicians think that side effects will be a barrier to wider GLP-1RA adoption. Only 8 percent of physicians said that they think side effects will be a barrier, showing that for many patients, the potential risk of side effects is outweighed by the medication benefits.
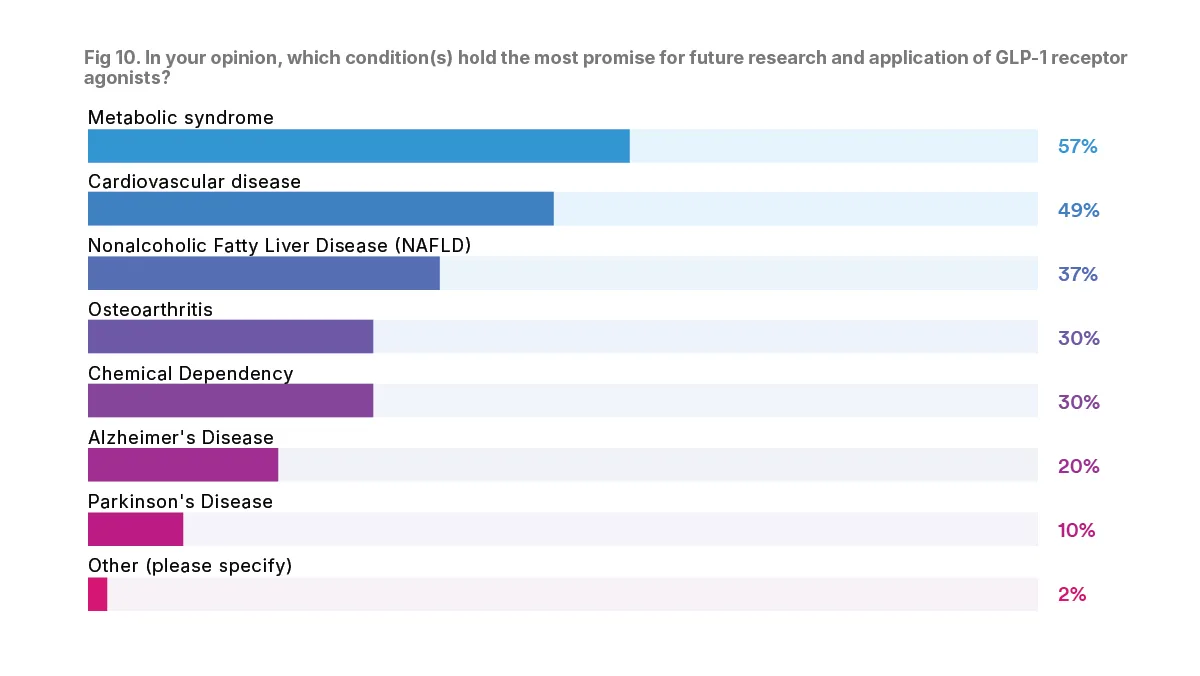
It is clear that in the future, GLP1RAs could be broadly applicable as a treatment for chronic diseases that impact many physician specialties. Basic research is still being done, and there may be many additional diseases that it will be able to treat.
Physicians were asked about which condition they believe holds the most promise for future research and application of GLP-1RAs. The majority, 57 percent, said that they think the future of GLP-1RA research is in metabolic syndrome. Another large percentage, 49 percent, said they believe the future of GLP-1RA research is in cardiovascular disease.
Other indications which physicians said could be the future of GLP-a research include nonalcoholic fatty liver disease (37 percent), osteoarthritis (30 percent), and chemical dependency (30 percent).
Each of these chronic conditions impacts millions of Americans, meaning GLP-1RAs could one day be the most common medication in the U.S.
Many physicians surveyed had unbridled enthusiasm about GLP-1 medications and their ability to treat non-diabetic and non-weight loss conditions.
“I think the stuff is great,” one physician responded. Another added that these medications “hold immense promise.”
Still, many physicians were reserved and waiting for more clinical data. “I think there is some potential benefit,” one physician said, “but I would like to see the long term data of how to keep these gains.”
According to another physician, “more long term research is needed. Time will tell if we are doing the right thing for our patients.”
Cure, a premier healthcare innovation campus and hub for entrepreneurs in New York City, conducted the GLP1RA Survey in collaboration with the Deerfield Institute, a division of Deerfield Management Company, an affiliate of Cure. Cure and Deerfield Institute conducted the survey in December 2024.
The survey received responses from 122 U.S.-based physicians with experience prescribing GLP-1RA medications, of whom 18 percent practiced addiction medicine and 20 percent each specialized in cardiology, gastroenterology, neurology and orthopedics. An industry-standard honorarium was provided to encourage thoughtful participation.
The majority of physicians have been in practice for more than 10 years: 13 percent practiced for fewer than 10 years, while 40 percent practiced for 10 to 19 years, 34 percent for 20 to 29 years, and 12 percent for more than 30 years.
Bergmann NC, Davies MJ, Lingvay I, Knop FK. Semaglutide for the treatment of overweight and obesity: A review. Diabetes Obes Metab. 2023; 25(1): 18-35. doi:10.1111/dom.14863.
Cummings JL, Atri A, Feldman HH, et al. evoke and evoke+: design of two large-scale, double-blind, placebocontrolled, phase 3 studies evaluating efficacy, safety, and tolerability of semaglutide in early-stage symptomatic Alzheimer’s disease. Alzheimers Res Ther. 2025;17(1):14. Published 2025 Jan 8. doi:10.1186/s13195-024-01666-7.
FDA Approves First Medication for Obstructive Sleep Apnea. FDA News Release. December 20, 2024.
Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse Effects of GLP-1 Receptor Agonists. Rev Diabet Stud. 2014 Fall-Winter;11(3-4):202-30. doi: 10.1900/RDS.2014.11.202.
GLP-1 Agonists. Cleveland Clinic. July 2023.
GLP-1-based therapy for obesity. Lasker Foundation. 2024.
GLP-1 drug liraglutide may protect against dementia. Alzheimer’s Association. July 30, 2024.
Holliday MW Jr, Frost L, Navaneethan SD. Emerging evidence for glucagon-like peptide-1 agonists in slowing chronic kidney disease progression. Curr Opin Nephrol Hypertens. 2024 May 1;33(3):331-336. doi: 10.1097/ MNH.0000000000000976.
Jain AB, Lorenzo L. GLP-1 RA Therapy for Alcohol Use Disorder? Medscape. September 9 2024.
Mahase E. GLP-1 agonists: US sees 700% increase over four years in number of patients without diabetes starting treatment. BMJ. 2024; 386 :q1645 doi:10.1136/bmj.q1645.
McCoy RG, Herrin J, Swarna KS et al. Effectiveness of glucose-lowering medications on cardiovascular outcomes in patients with type 2 diabetes at moderate cardiovascular risk. Nat Cardiovasc Res 3, 431–440 (2024). doi: 10.1038/s44161-024-00453-9.
Mehdi SF, Pusapati S, Anwar MS, Lohana D, Kumar P, Nandula SA, Nawaz FK, Tracey K, Yang H, LeRoith D, Brownstein MJ, Roth J. Glucagon-like peptide-1: a multi-faceted anti-inflammatory agent. Front Immunol. 2023 May 17;14:1148209. doi: 10.3389/fimmu.2023.1148209.
Meurot C, Jacques C, Martin C, Sudre L, Breton J, Rattenbach R, Bismuth K, Berenbaum F. Targeting the GLP-1/ GLP-1R axis to treat osteoarthritis: A new opportunity? J Orthop Translat. 2022 Feb 25;32:121-129. doi: 10.1016/j. jot.2022.02.001.
Mitchell JL, Lyons HS, Walker JK, Yiangou A, Grech O, Alimajstorovic Z, Greig NH, Li Y, Tsermoulas G, Brock K, Mollan SP, Sinclair AJ. The effect of GLP-1RA exenatide on idiopathic intracranial hypertension: a randomized clinical trial. Brain. 2023 May 2;146(5):1821-1830. doi: 10.1093/brain/awad003.
Montero A, Sparks G, Presiado M, Hamel L. KFF Health Tracking Poll May 2024: The Public’s Use and Views of GLP-1 Drugs. KFF. May 2024.
Nevola R, Epifani R, Imbriani S, Tortorella G, Aprea C, Galiero R, Rinaldi L, Marfella R, Sasso FC. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int J Mol Sci. 2023 Jan 15;24(2):1703. doi: 10.3390/ijms24021703.
Page S. How do GLP-1 weight loss drugs affect the liver? Michigan Medicine. University of Michigan. August 14 2024.
Quddos F, Hubshman Z, Tegge A. et al. Semaglutide and Tirzepatide reduce alcohol consumption in individuals with obesity. Sci Rep 13, 20998 (2023). doi: 10.1038/s41598-023-48267-2.
Reddy S. They Thought Ozempic Would Help Them Lose Weight. It Didn’t Work. The Wall Street Journal. April 2024.
Salie, Faith. Can GLP-1 Weight Loss Drugs Protect the Heart? Health Matters Podcast, New York-Presbyterian Hospital, November 2024.
Sango K, Takaku S, Tsukamoto M, Niimi N, Yako H. Glucagon-Like Peptide-1 Receptor Agonists as Potential Myelination-Inducible and Anti-Demyelinating Remedies. Front Cell Dev Biol. 2022 Jul 6;10:950623. doi: 10.3389/ fcell.2022.950623.
Shao S, Zhang X, Xu Q, Pan R, Chen Y. Emerging roles of Glucagon like peptide-1 in the management of autoimmune diseases and diabetes-associated comorbidities. Pharmacol Ther. 2022 Nov;239:108270. doi: 10.1016/j.pharmthera.2022.108270.
Shao S, Zhang X, Xu Q, Pan R, Chen Y. Emerging roles of Glucagon like peptide-1 in the management of autoimmune diseases and diabetes-associated comorbidities. Pharmacol Ther. 2022 Nov;239:108270. doi: 10.1016/j.pharmthera.2022.108270.
Zhao X, Wang M, Wen Z, Lu Z, Cui L, Fu C, Xue H, Liu Y, Zhang Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front Endocrinol. 2021 Aug 23;12:721135. doi: 10.3389/fendo.2021.721135.
CONTACT US:
Email: info@cureexperience.com
345 Park Avenue South
New York, NY 10010
About Cure: Cure is a healthcare innovation campus in the heart of New York City that features laboratory and business facilities, a collaboration residency, office space and premium event venues, including an education center, conference center, and iconic rooftop facility, as well as tools, mentoring, networking, and other assistance to members of its ecosystem. Cure houses on-campus startups and established companies. Residents regularly create synergies and collaborative partnerships with peer organizations across the spectrum of healthcare, from academic or private to non-profit or government, and focus on diagnostic, device, drug or vaccine discovery, development and production as well as care delivery and public health. Cure also offers industry-leading event programming focused on critical health topics. Cure’s mission is to foster and accelerate advances in health.







