Overview
Robert C. Green Director, Genomes2People and Precision Population Health Professor at Harvard Medical School, co-leads the first NIH-funded trials of genetic sequencing adults (MedSeq), newborns (BabySeq) and active-duty military personnel (MilSeq).
What is your risk for a genetic disease?
Do you really want to know your risk of genetic disease? Cure asked Robert C. Green MD, MPH, why we such screenings might be more routine for our healthcare in the near future.
Green, Director, Genomes2People and Precision Population Health Professor, Harvard Medical School, is recognized for his research and policy work to accelerate genomic and precision medicine. He also co-leads the first NIH-funded randomized trials of sequencing in adults (MedSeq), newborns (BabySeq) and active-duty military personnel (MilSeq).
Cure spoke with Green about progress and controversy in genomic screening, and his new company Nurture Genomics, which offers genetic screening for babies and children to help provide meaningful health insights to their parents and healthcare providers.
Why do you think it’s time for population genomics to expand into the public consciousness and the healthcare workstream?
Green: At some point, genomics has to be part of our healthcare. There are very affordable screening tests available now. Ask yourself this: Why doesn't my doctor ask me to get some sort of screening test? It's because they don't know about it. It's because of the fear factor, the concern about privacy discrimination, because there are not enough experts — all these reasons we know about. But the truth is, it saves lives.
If we can simply, realistically communicate the narrative that we can prevent illness, and we can treat people early, then we can have people who live longer. The ink that's being spilled over longevity these days! The best way to live a long time is not to die early. So, let's surveil those individuals who are at high risk for cancer. Let's identify those people who are at high risk for sudden cardiac death.
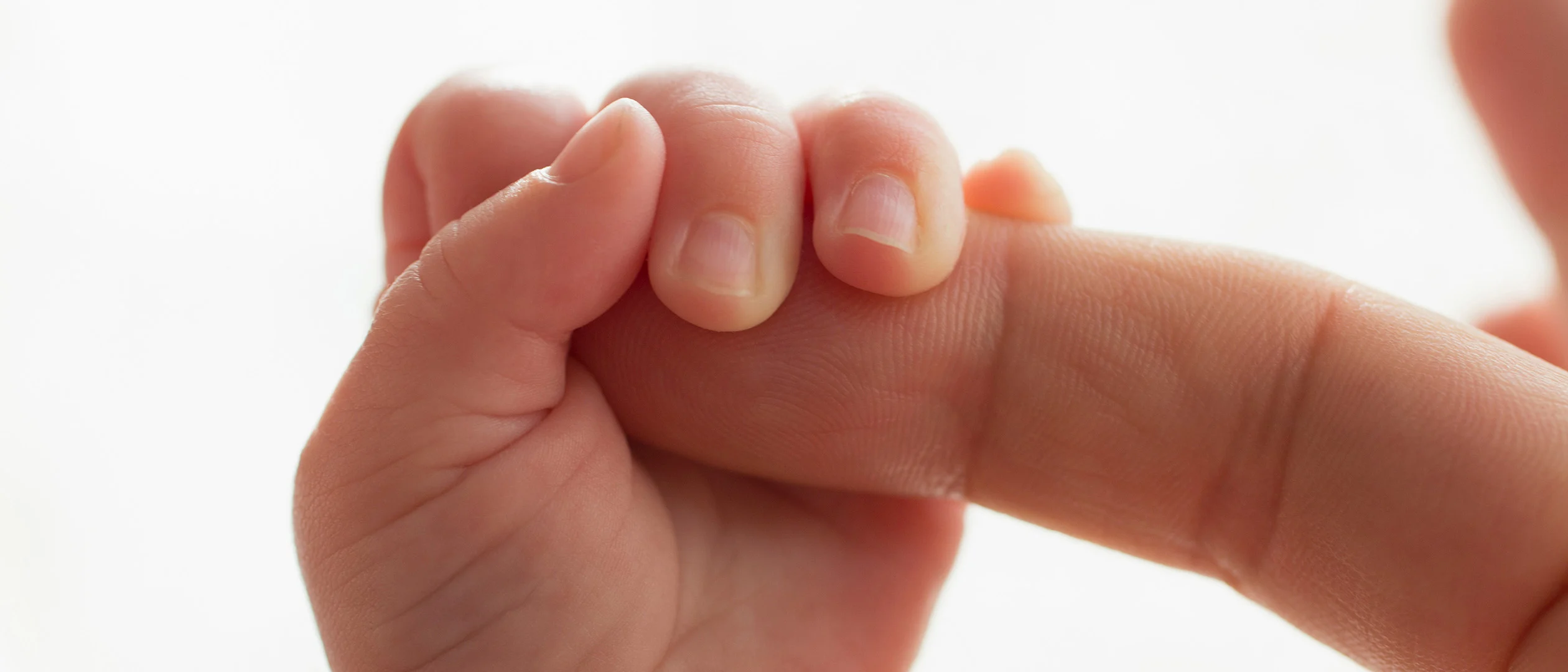
One of your great passions is the BabySeq Project, to screen healthy infants using whole genome sequencing. How is this information being received by physicians and parents?
Green: Many people would say they don't want to know, but I predict that in 10 years, 15 years, 20 years, everyone will want to know. Many diseases that we consider rare are not rare in the aggregate. Many metabolic conditions, many biochemical conditions, many early cancers are hereditary in the sense that they're driven by a known mutation. And we're not looking for them. At the time a child is born you can get the whole blueprint. There are four different categories where you could predict, stratify risk, and potentially prevent, and you could start that as a child:
Single gene diseases. These are huge — 20 percent of your audience is carrying a mutation for single gene disease, and they don't even know it. Not all of those will make them sick, but some of them will. And they would be better off if they knew it.
Recessive carrier traits. A bunch of people reading this are going to have children, and you don't know which recessive carrier trait you have. If you and your partner knew, you'd be able to avoid some of the devastating childhood diseases.
Medicine Biomarkers. Many people are going to be on medicine throughout their lives. If you had the pharmacogenomic markers to know which medicines were good for you and which ones were going to cause side effects, you would be healthier when you got those medicines.
Polygenic risks. There's a whole new category called polygenic risk scores, which means you add up thousands of low impact markers and you define which folks are going to have a very high risk of common conditions like common cancers, type 2 diabetes, atrial fibrillation, and so forth.
In BabySeq, in the academic world, we did look for adult-onset conditions in the babies, and we found some babies with breast and ovarian cancer mutations. It’s going to be very controversial for a while to try to bring adult conditions into a child’s life.
I am sure that you also get people who are a little bit scared, asking what are you going to do with this information? The moment the Human Genome Project started, ethical, legal, social implications side projects also began.
Green: Absolutely, and an important vertical in our MedSeq and BabySeq projects, both of which have been led by Amy McGuire, JD, PhD, at Baylor, has been an entire team thinking about and focusing on the legal, ethical, social implications. Not that we solve those issues, but we have tried to think deeply about them, and also disparity issues. How do you try to make sure that novel technologies are distributed equally in both research and practice?
Let’s not exceptionalize genomics so much that we can’t use it for good. Privacy and discrimination can be used against you for any medical issue. What is your sexuality? Are you HIV positive? Genomics is not special. Let’s pay attention to bias, discrimination, privacy — let’s not be cavalier. But let’s not exceptionalize it to the point where we can’t use the lifesaving value.
You have started a new company, called Nurture Genomics. Can you tell us what the company is working on?
Green: Nurture Genomics is a digital health platform that will make newborn and childhood sequencing available to everyone. I'm excited to say that the platform is open for a few states — genomic screening for over 400 genes that are all associated with treatable genetic conditions in children.
Can adults get sequenced?
Green: All the big labs are pivoting into consumer-facing panels. But children are kind of a new area, and it's also a different psychology. People will do things for their children, for the next generation, that they won't do for themselves. We think maybe this is a way to bring genomics into a family.







