Overview
The FDA’s new plan to phase out many animal studies is already reshaping drug R&D as startups and pharma teams adopt digital and human-cell platforms.
Organ-on-a-chip, AI toxicity models and organoids race to meet agency’s call for “human-relevant” data
Scientists experiment on millions of animals every year to determine what new medicines might be safe and effective enough to test in humans. For all the successes of this decades-old model, it has its flaws. Beyond the ethical considerations, most therapies that work in mice or other lab-bred animals don’t end up working in people.
Now, the FDA wants to bring medical research into the 21st century by phasing out animal research. The regulatory agency’s plan calls for options including digital twins, existing real-world data, artificial intelligence and other tools. Earlier this month, the FDA published a roadmap on how this change will occur.
“This initiative marks a paradigm shift in drug evaluation and holds promise to accelerate cures and meaningful treatments for Americans while reducing animal use,” said FDA Commissioner Martin A. Makary, MD, MPH, in a news release. “By leveraging AI-based computational modeling, human organ model-based lab testing, and real-world human data, we can get safer treatments to patients faster and more reliably, while also reducing R&D costs and drug prices. It is a win-win for public health and ethics.”
How policy opened the door for new test methods
Animal testing before human trials has been mandated by Congress since 1938, after a pharmaceutical company released a product that caused a mass poisoning event. The mandate was lifted in 2022 with the FDA Modernization Act 2.0.
The FDA’s new roadmap encourages drug developers to submit New Approach Methodologies (NAMs) data—microphysiological systems (MPS), AI toxicity models, organoids and human real-world evidence—alongside or in place of legacy animal studies.
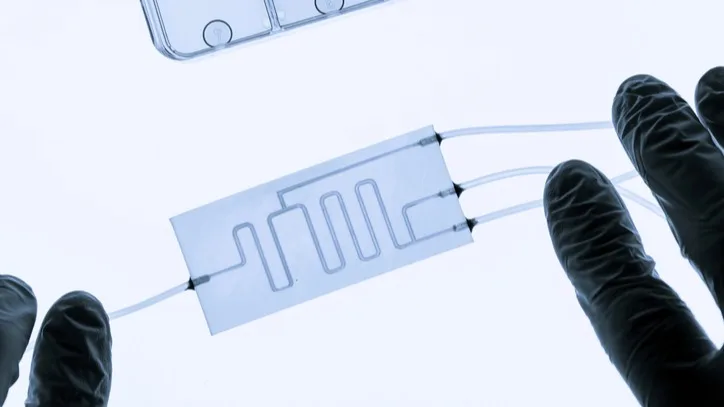
Organ-on-a-Chip
Organ-on-a-chip devices aim to replicate the micro-architecture of human organs and generate continuous readouts. Several companies have been testing these models and publishing research on how they perform in the real-world, showing better results than traditional methods. Emulate’s Liver-Chip has been accepted into a FDA pilot program aimed at qualifying the platform to predict drug-induced liver injury.
“Traditional animal and reductionist models are limited because they simply do not capture the complexity of the human body,” said Lorna Ewart, PhD, Chief Scientific Officer of Emulate, in a statement. “With Organ-Chips, we can explore biological mechanisms of health and disease in a setting that more closely mirrors human physiology.”
Digital Toxicity Models
Another company, Schrödinger, developed a digital lab program to predict toxicology risk early in drug discovery, and received funding from the Bill & Melinda Gates Foundation, with a goal to flag off-target risks early, before compounds ever reach animals or chips.
“The science has evolved — today, modeling and simulation can offer human-relevant insights that not only complement but in many cases can begin to replace traditional animal studies,” said Shawn O’Connor, Chief Executive Officer of Simulations Plus, in a statement. “This roadmap is an important step toward a future where safer, faster, and more sustainable drug development is possible.”
Early validation and remaining hurdles to improve drug testing
Organs-on-chips could make drug testing more accurate, but hurdles remain. A 2024 Nature Communications perspective points to major barriers, including inconsistent device performance, lack of standardized methods, and limited head-to-head comparisons with animal models.
Regulators also need more evidence before replacing traditional testing. Without clear standards and validation, the technology will stay an add-on rather than a true alternative.
“The rapid development of Organs on Chips, primarily driven by interest from pharmaceutical companies, has led to a need for industry standards for the field,” researchers wrote in the Nature Perspective paper. “However, standardization cannot stifle innovation. Properly addressing the translational barriers for various applications is key for Organs on Chips to reach their ultimate potential.”
What happens next for NAMs development
The FDA and NIH will co-host a workshop this fall to hammer out validation criteria and pick participants for a monoclonal-antibody pilot that relies primarily on NAMs. Guidance updates will follow in phases through 2026.
If the pilot succeeds, thousands of dogs, primates and rodents could be spared annually, and the first therapy supported mainly by chip-only safety data might reach human trials within three years.
“For patients, it means a more efficient pipeline for novel treatments,” FDA Commissioner Makary, said. “It also means an added margin of safety, since human-based test systems may better predict real-world outcomes. For animal welfare, it represents a major step toward ending the use of laboratory animals in drug testing.








